Metabolic Dysfunction–Associated
Steatohepatitis (MASH)
Steatohepatitis (MASH)
A Chronic Liver Disease
MASH is a leading cause of liver-related mortality and an increasing burden on global healthcare systems.
The Role of Thyroid Hormone Receptor β (THR-β) in MASH
In MASH, reduced signaling via THR-β exacerbates hepatic mitochondrial dysfunction, inflammation, and fibrosis.
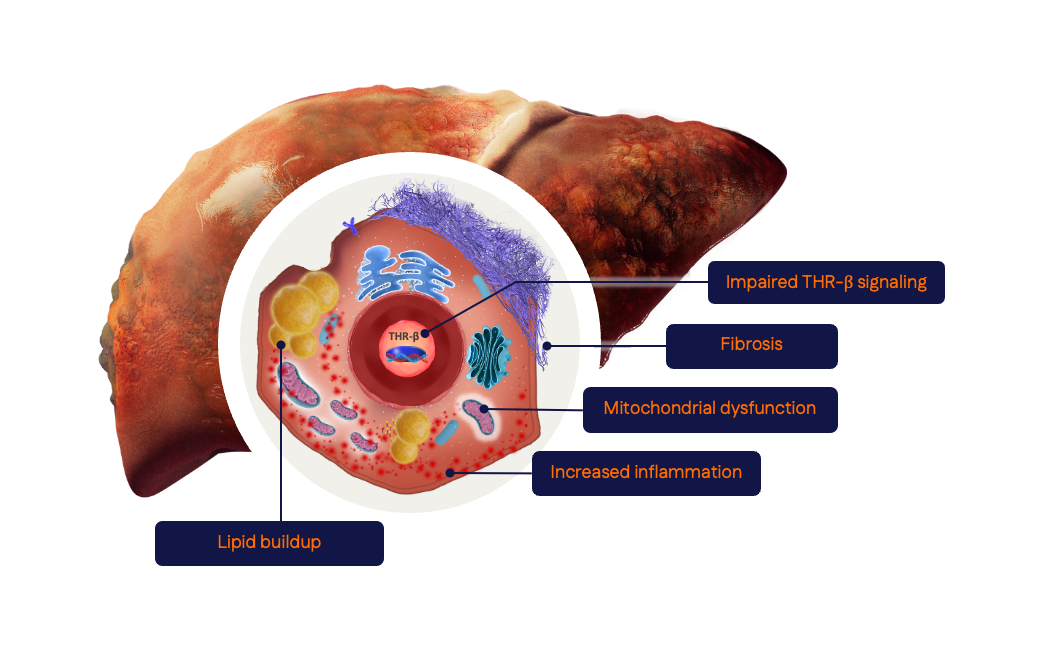

The Role of Thyroid Hormone Receptor β (THR-β) in MASH
In MASH, reduced signaling via THR-β exacerbates hepatic mitochondrial dysfunction, inflammation, and fibrosis.
Our Research
MAESTRO Clinical Development Program
Our clinical development program focuses on a thyroid hormone receptor-β (THR-β) agonist being studied for the treatment of MASH.
Publications
View key resmetirom and clinical publications along with recent congress presentations.
