N Engl J Med. 2024;390(6):497-509. doi:10.1056/NEJMoa2309000
A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis
Stephen A Harrison, Pierre Bedossa, Cynthia D Guy, Jörn M Schattenberg, Rohit Loomba, Rebecca Taub, Dominic Labriola, Sam E Moussa, Guy W Neff, Mary E Rinella, Quentin M Anstee, Manal F Abdelmalek, Zobair Younossi, Seth J Baum, Sven Francque, Michael R Charlton, Philip N Newsome, Nicolas Lanthier, Ingolf Schiefke, Alessandra Mangia, Juan M Pericàs, Rashmee Patil, Arun J Sanyal, Mazen Noureddin, Meena B Bansal, Naim Alkhouri, Laurent Castera, Madhavi Rudraraju, Vlad Ratziu 1; MAESTRO-NASH Investigators
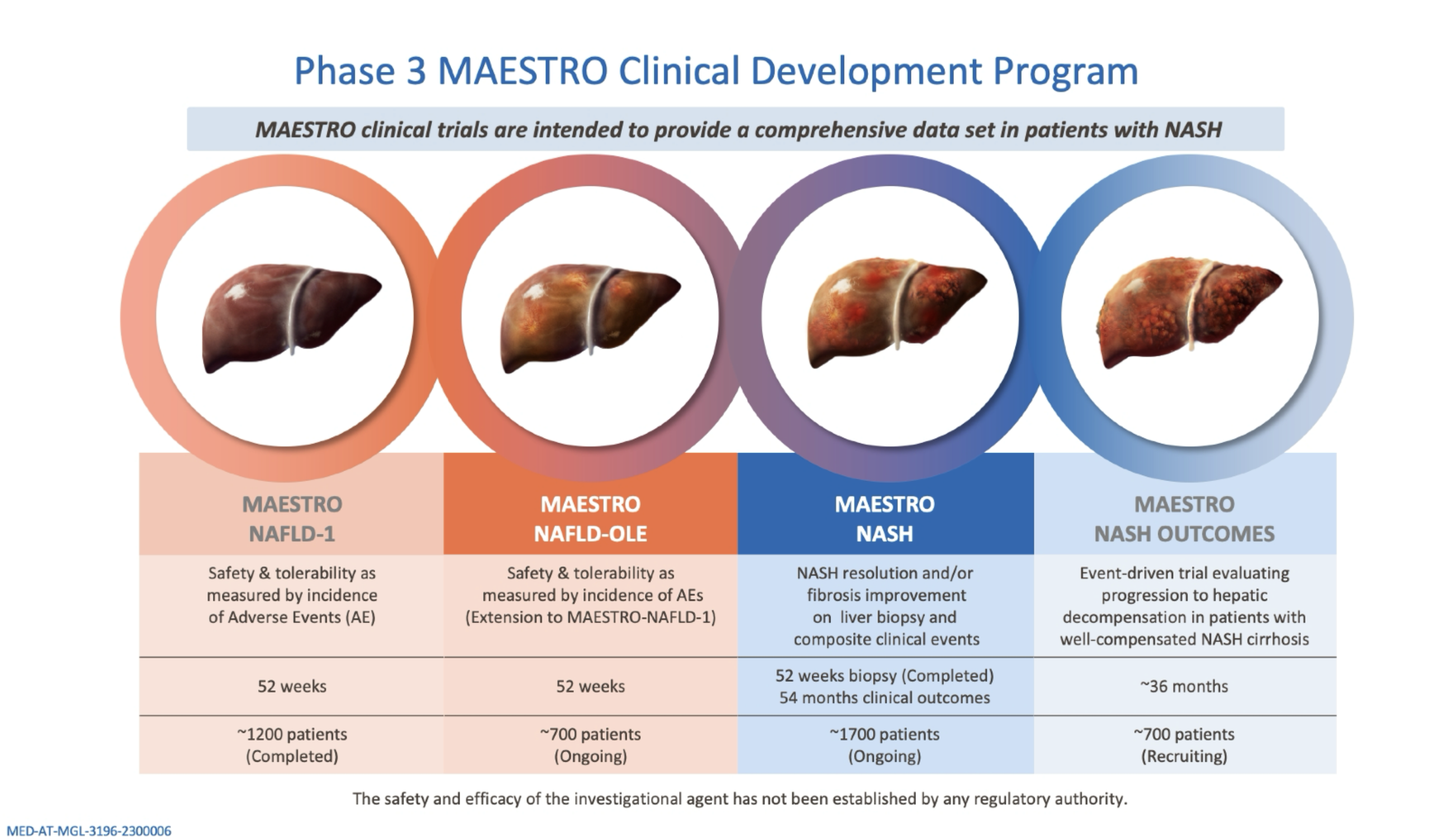
NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; OLE, open-label extension.
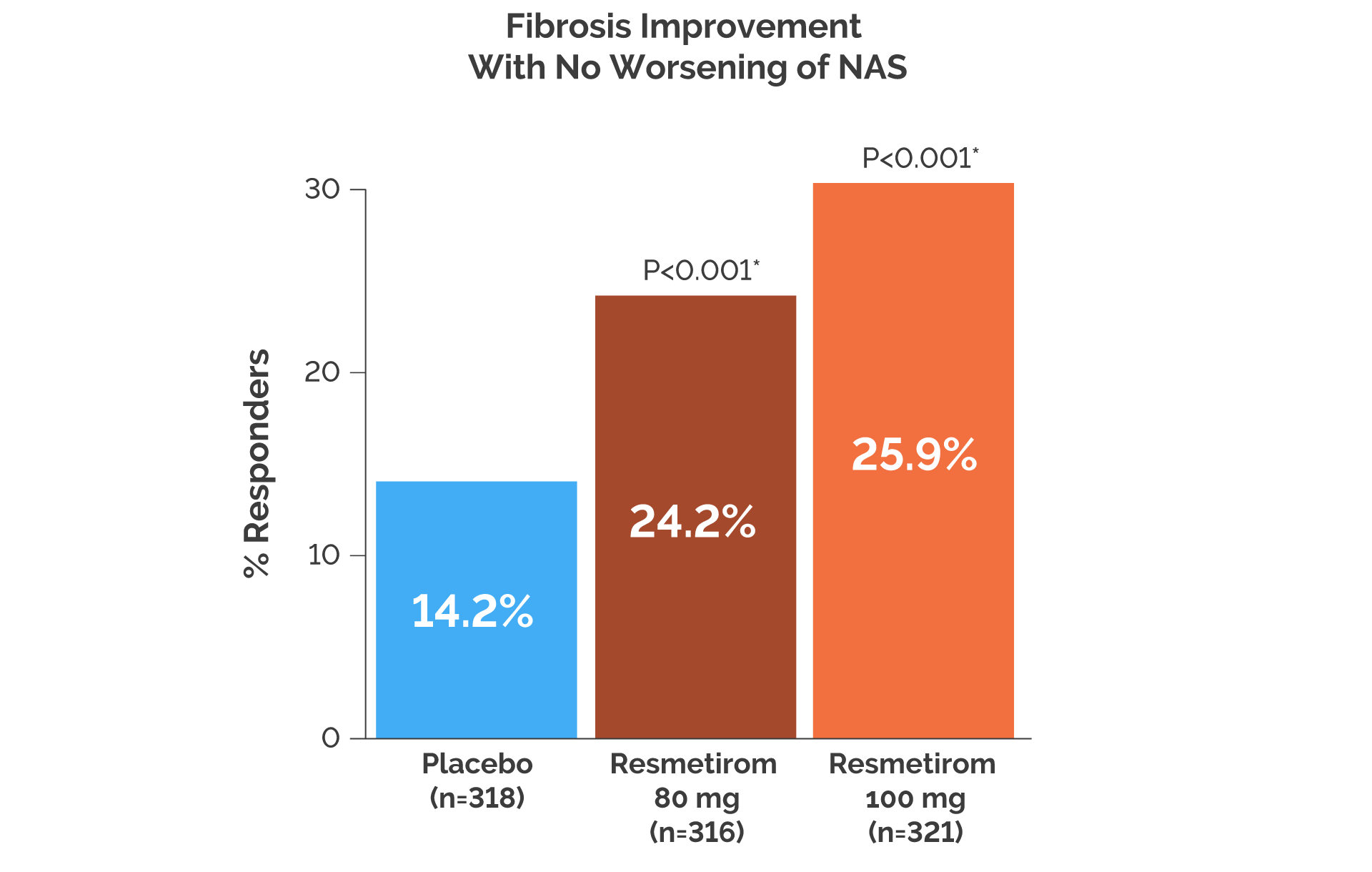
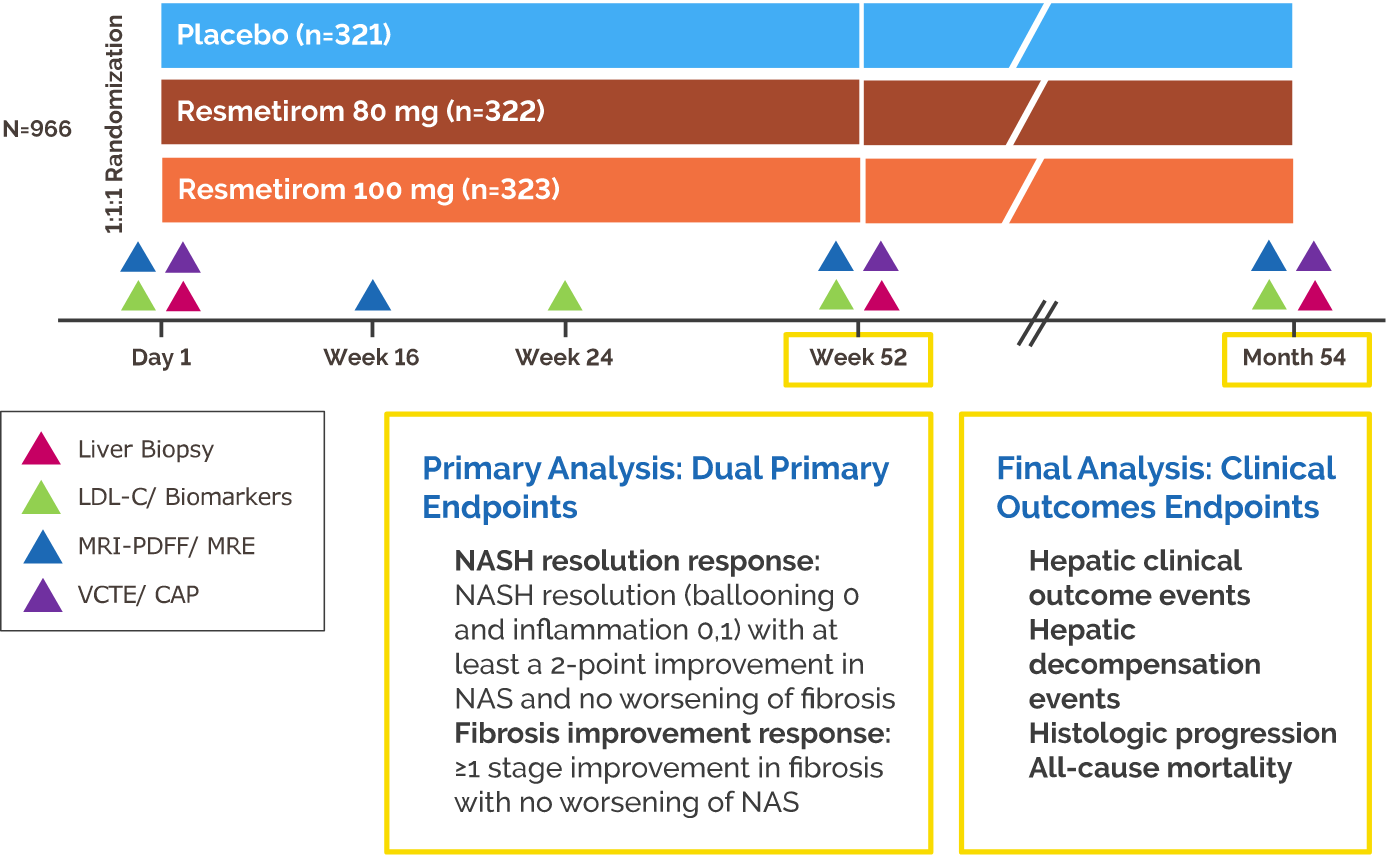
- At Week 52, NASH resolution and fibrosis improvement were achieved in significantly more patients treated with resmetirom versus placebo (P<0.001)
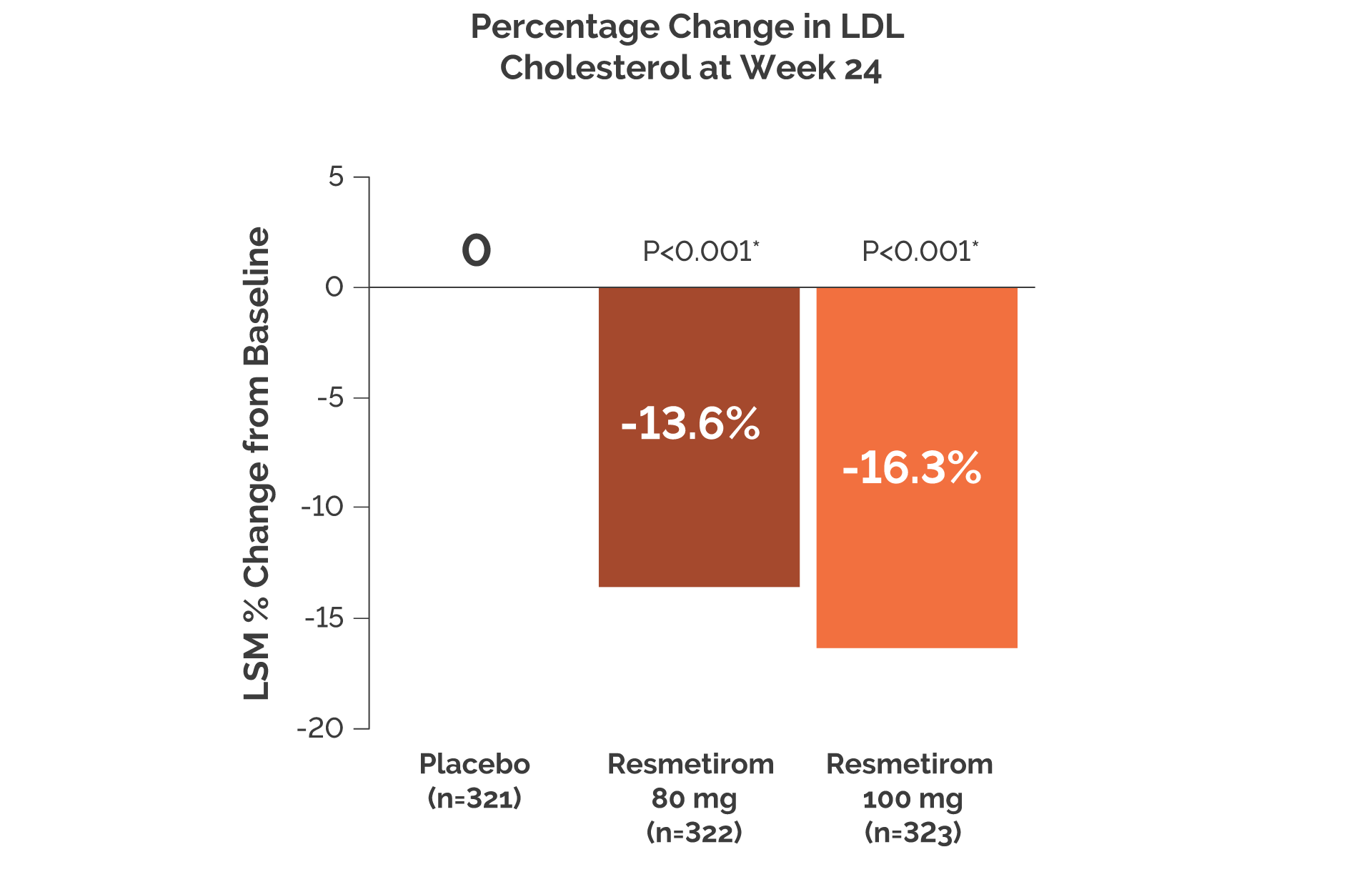
- At Week 24, LDL-C was reduced from baseline with resmetirom versus placebo (P<0.001)
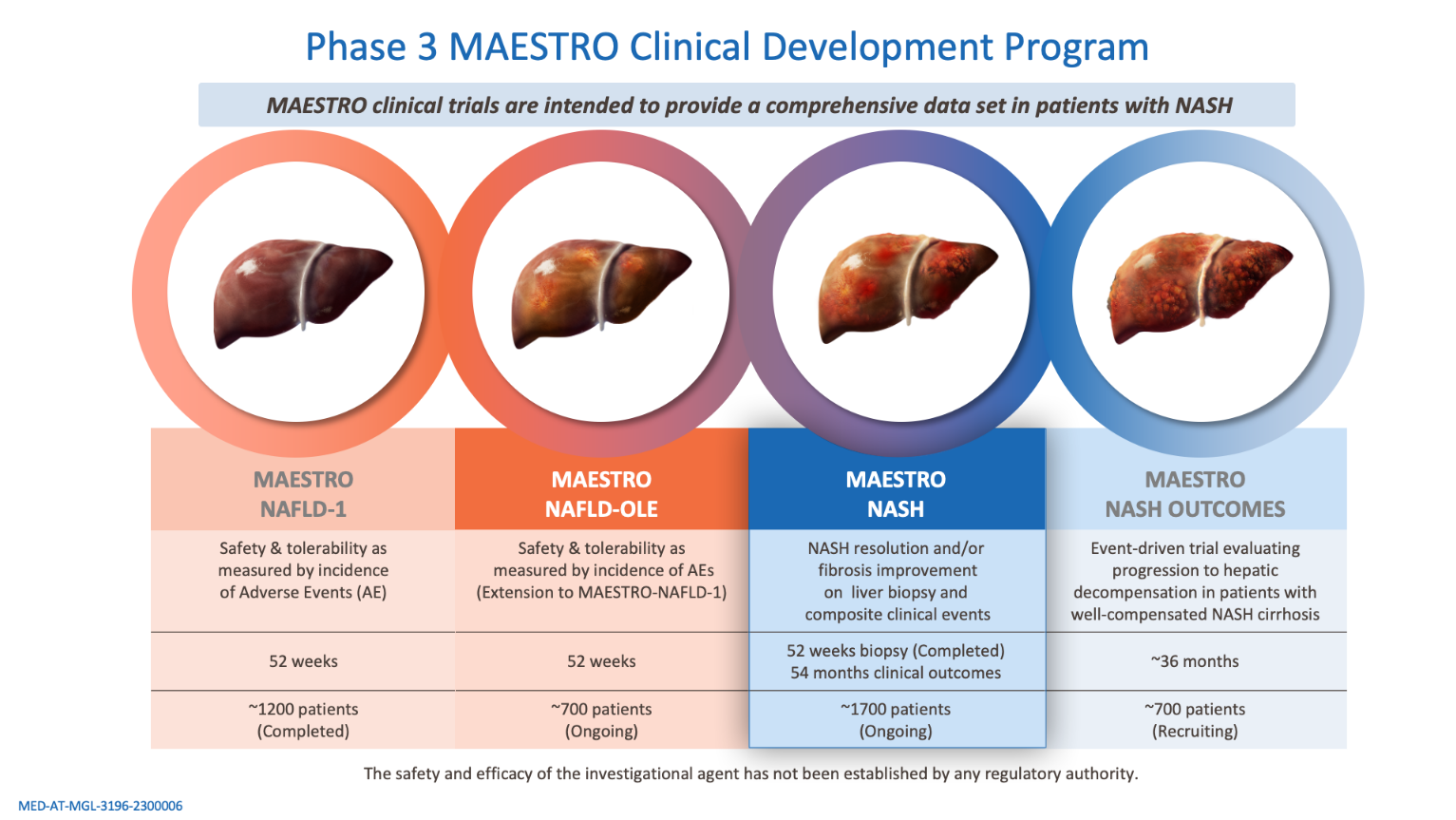
NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; OLE, open-label extension.
- At Week 52, NASH resolution and fibrosis improvement were achieved in significantly more patients treated with resmetirom versus placebo (P<0.001)
- At Week 24, LDL-C was reduced from baseline with resmetirom versus placebo (P<0.001)
NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; OLE, open-label extension.
- The results of additional biopsy endpoints and sensitivity analyses were generally supportive of the results of the primary analyses of the dual primary endpoints
- At Week 52, the proportion of NAS improvement and fibrosis responders was numerically greater with resmetirom compared with placebo
LDL-C, low-density lipoprotein cholesterol; NAFLD, nonalcoholic fatty liver disease; NAS, NAFLD activity score; NASH, nonalcoholic steatohepatitis.
- At Week 24 and Week 52, key lipids/lipoproteins were reduced from baseline with resmetirom compared with placebo
ApoB, apolipoprotein B; TG, triglycerides; BL, baseline; LDL-C, low-density lipoprotein cholesterol; Lp(a), lipoprotein (a).
- Among patients with high baseline ALT (≥30 IU), levels of ALT were reduced with resmetirom compared with placebo
ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transferase; IU, international units.
*Data are for patients with baseline ALT ≥30 IU.
- Incidence of serious AEs was similar across treatment groups
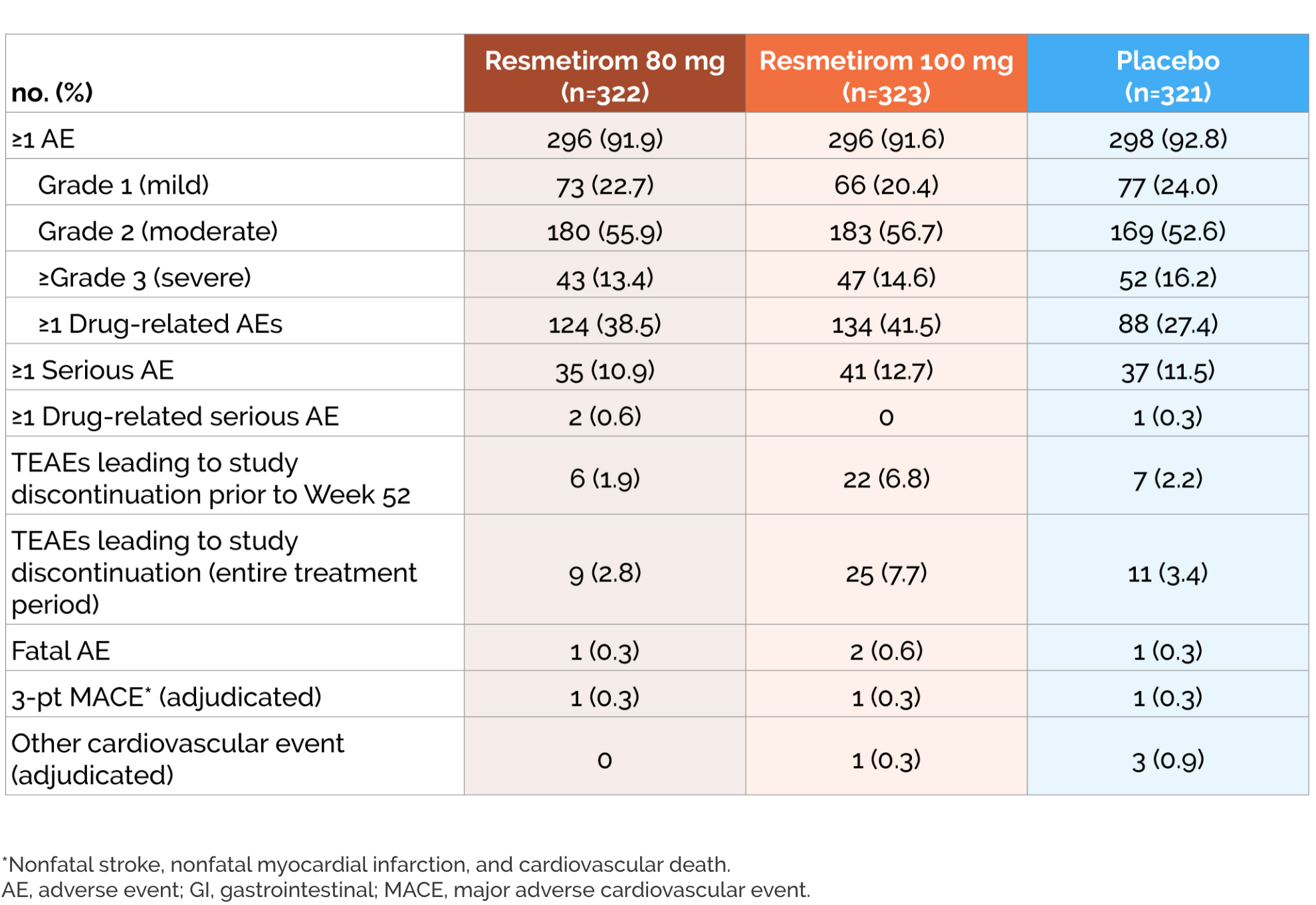
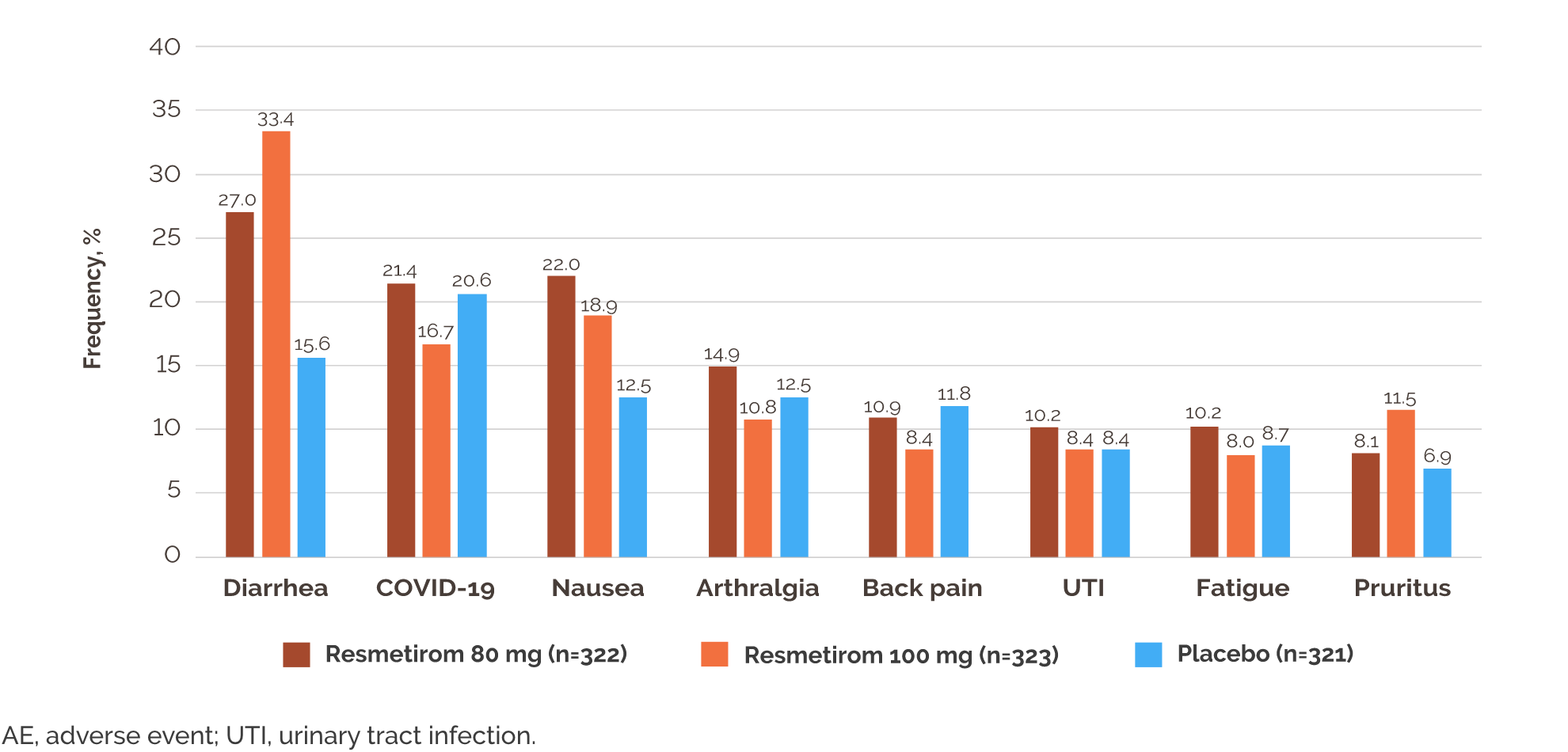
- Diarrhea and nausea were more frequent with resmetirom